Rheumatology
Members of the Research Team:
Dr Oksana Kehoe (Lead) and PhD students Rebecca Davies, Anais Makos and Henry Barrett
Clinical Support:
Dr Roshan Amarasena (Lead), Dr Ayman Askari, Dr Julia Flint and Dr Rameez Arif.
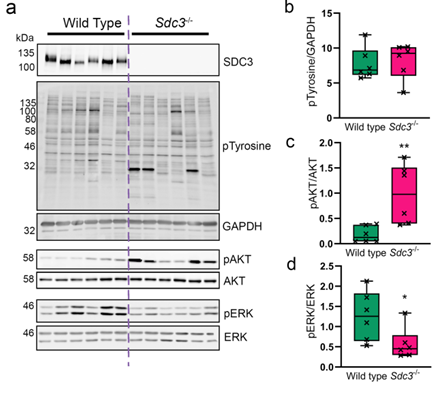
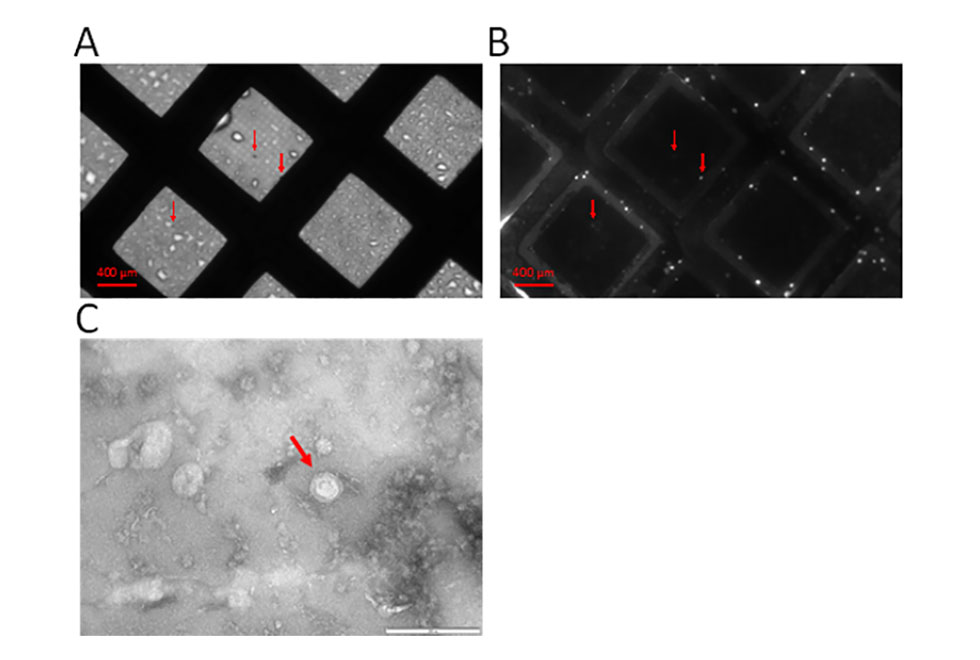
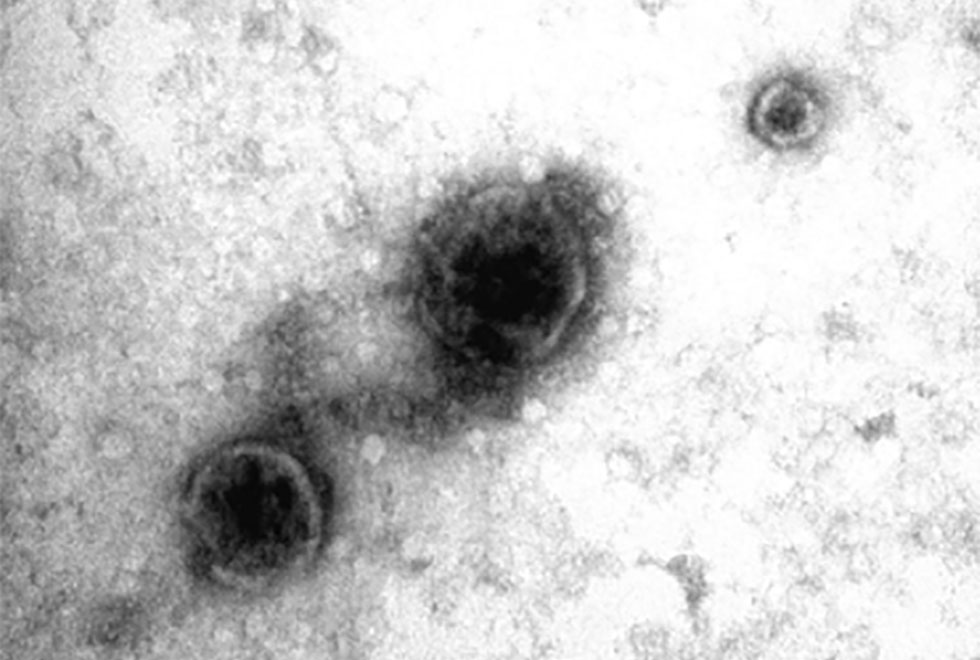
Our team has continued to be active this year carrying out basic science work into understanding mechanisms of inflammatory arthritis diagnosis, progression and possible treatments including mesenchymal stem cells, regulatory T cells and their extracellular vesicles. We also try to find out how stem cells can be “encouraged” to perform better in aging and in disease such as arthritis.
We are very fortunate in RJAH to be able to work closely with clinicians who themselves are very interested in research.
We have been publishing our findings in the International Journal of Molecular Sciences, Inflammopharmacology, Cytotherapy and Annals of the Rheumatic Diseases and have presented our work at several national and international meetings such as the 8th Malaysian Tissue Engineering and Regenerative Medicine Scientific Meeting, March 2022; the International Society for Extracellular Vesicles Annual Meeting, May 2022, Lyon, France (Figure 1); TERMIS EU 2023, March 2023, Manchester, UK; the International Society for Extracellular Vesicles Annual Meeting, May 2023, Seattle, USA; EULAR 2023 Congress, May 2023, Milan, Italy.
We are very grateful to the Orthopaedic Institute Charity, Oswestry for their continuous support.
Figure 1– Dr Oksana Kehoe and Becky Davies at the ISEV Annual Meeting in Lyon, France, May 2022.
Rheumatology Projects