Developing a Cell Therapy delivery system for Chronic Wounds
Angus Armstrong Twigg, Mateus Bernardo Harrington, Naveen Kumar, Joy Chowdhury, Aheed Osman, Srinivasa Budithi and Karina Wright
Funded by Orthopaedic Institute
Pressure ulcers (PrUs), are a frequent and serious complication of spinal cord injury (SCI), with subsequent treatment costing the NHS up to £374 per day per individual. The partial or complete loss of local sensation following SCI makes these patients one of the highest at-risk populations for PrU development. Treating PrUs effectively requires a multifaceted approach, including close monitoring of the wound and appropriate nutrition.
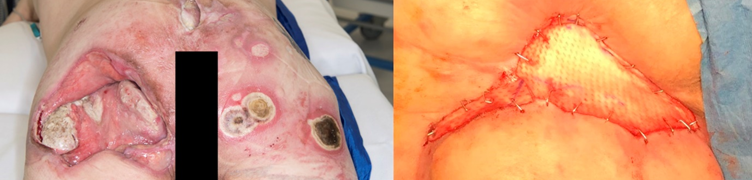
Earlier this year we were the first group in the UK to apply an acellular dermal allograft to a very severe PrU (Figure 2A). This patient has recovered and been safely discharged. The team is currently assessing whether seeding these dermal grafts with allogeneic human dermal fibroblasts or bone marrow stromal cells will enhance PrU healing. We have characterised polydactyly-derived juvenile primary dermal fibroblasts (JPDFs) by flow cytometry, with stimulation from either Interferon-γ (IFN-γ) for 24h at 25ng/ml (inflammatory stimuli) or lipopolysaccharide for 24h (microbial stimuli) at 10ng/ml, alongside matched cultures without stimulation, and assessed their biocompatibility with a commercial acellular dermis (Allosource®) (Figure 2B). Histological analysis of the seeded grafts revelled the cells had adhered and proliferated on the surface of the dermis.
Together this data suggests that we have begun to establish an effective cell delivery system for PrUs, which could be used to enhance cell repopulation and revascularisation of the wound bed.
Figure 2: Allograft solutions for chronic pressure ulcers (PrUs). A: Photograph of the first PrU case to be treated with this graft in the UK (left panel illustrates PrUs on admission (May-2018), right panel shows the dermal allograft secured to the largest PrU (Applied November-2018). B: Histological analysis of the allograft (left panel under polarised light and right panel with H&E staining.