Biomechanics Lab
Head of Research: Dr. Jan Herman Kuiper
Orthopaedic interventions
Collaborators: Mr Simon Pickard, Mrs Soha Sajid, Miss Emily Day, Dr Bernhard Tins, Miss Mara Muellensiefen, Mrs Kelly Campbell, Dr Shailesh Naire
Comparing two suture techniques to join flexor tendons
Tendon grafting and tendon transfer surgery are common hand procedures at our hospital. Tendon grafting can be necessary following a severe tendon injury and involves replacing part of the patient’s tendon with a donor tendon. Tendon transfer surgery can be used following nerve palsy, where the loss of control over a muscle has caused lost movement and function. Tendon transfer relocates the insertion of a functioning muscle-tendon unit to the tendon whose muscle control was lost, thus restoring function and movement. Both types of surgery rely on joining two tendons, normally using some form of suturing.
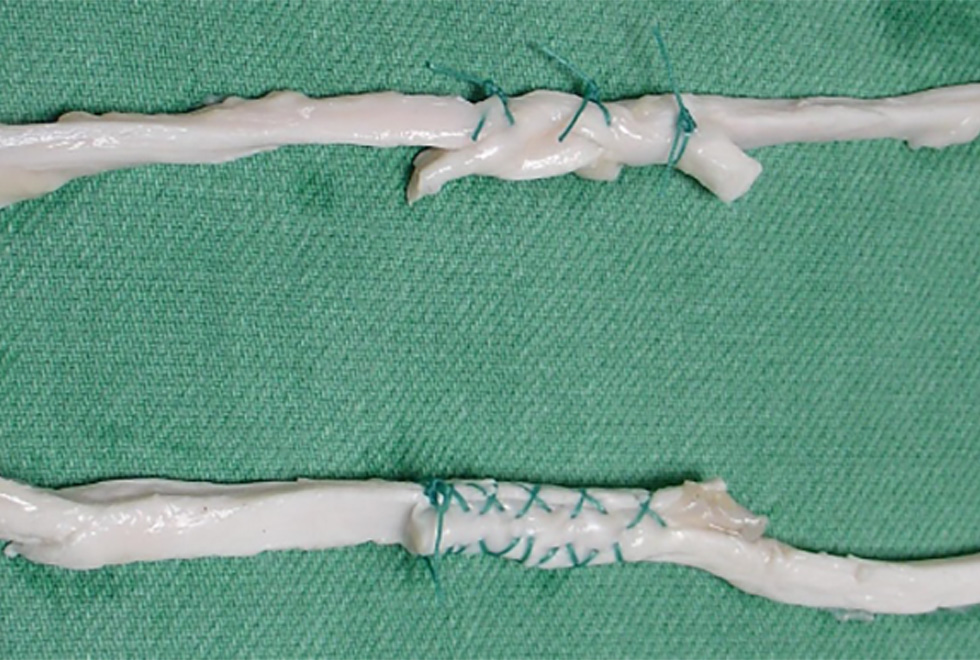
Immediate movement of the hand following surgery is important as it allows patients to start rehabilitating and adapt to the graft or transfer. Early rehabilitation prevents adhesions, gives earlier restoration of function and eventually results in a stronger tendon with improved vascularity. Of course the repair needs to be strong enough to withstand the mechanical loads during rehabilitation. Traditionally, tendons are joined using the Pulvertaft weave (PTW) technique. This relatively complicated technique involves first weaving the end of one tendon through slits in the end of the other tendon, before suturing them. An alternative is side-to-side (STS) repair, which involves simply laying the ends of the two tendons side-by-side and then suture them together. Several studies have compared the strength of PTW and STS and found that STS is stiffer and has a higher load to failure. However, PTW seems to work under physiological loading conditions, which are typically low intensity cyclic loads.
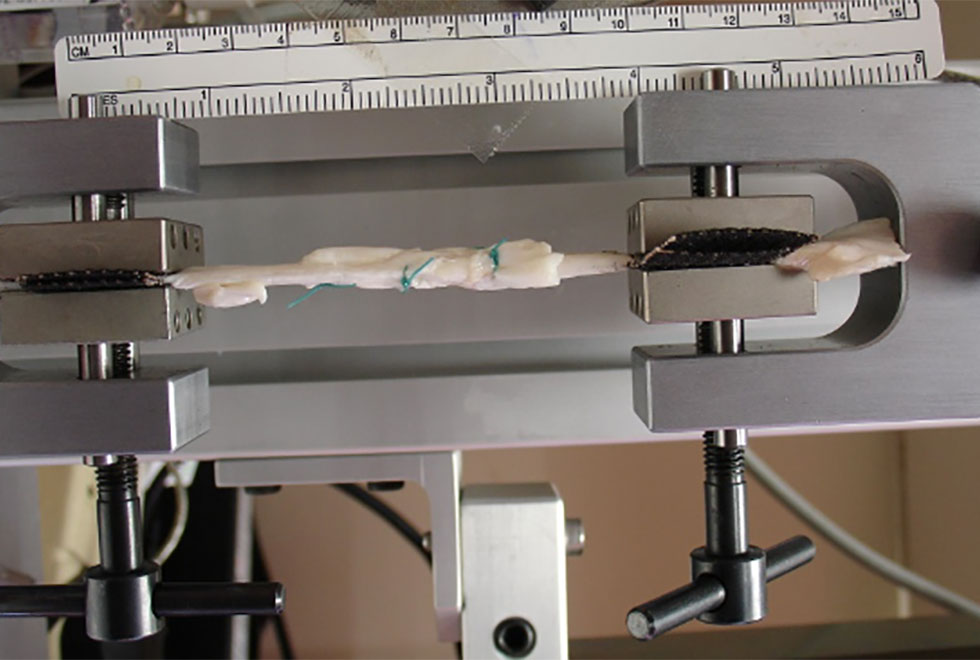
We therefore performed an experiment to find out how tendons joined using either of the repair methods behave when loaded cyclically at physiological levels. For this we used flexor tendons from pigs, which we cut through and then joined using either of the techniques (Figure 1). We then placed the samples in our materials testing machine (Fig. 2) where we first loaded them cyclically to mimic average rehabilitation loads measured in humans. Next we loaded them cyclically using the largest rehabilitation load measured in humans. If they had not failed at this stage, we pulled them slowly apart to find how much force they would take before failing. We considered them failed if the elongation of the tendon was above 25mm.
We found that the tendons joined using the STS technique were better at resisting cyclic loading at average rehabilitation loads. The repair elongated less and the increase of elongation after each load cycle was less. Nevertheless, all samples joined using either technique were strong enough to resist the average physiological load. However, when we applied the largest load measured in humans, only 4 out of 10 samples joined using the PTW technique stayed intact, whereas all 10 samples joined using the STS technique did. Clearly, once the rehabilitation loads reach the upper range of those measured, the PTW may not be strong enough.
Given these results, it should not surprise that the mean force to reach 25mm of elongation was much (four times) larger for the STS samples than for the PTW samples. We therefore concluded that although the PTW joining technique is probably sufficient strong for the average patient, some patients will generate large enough forces to damage the connection. Therefore the STS technique is the safer choice. Moreover, the STS technique is less complicated.
Phantoms for CT scanners
Being able to replace painful hips and knees by artificial joints is one of the success stories in orthopaedics. It is therefore no surprise that the main clinical activity of our hospital is joint replacement surgery. However, over a period of 20 years between 15 and 20% of the implants need revision, mainly because they have loosened and caused resorption of the surrounding bone.
Traditionally, radiologists and clinicians use X-rays to decide if there are bone defects around a prosthesis and how much bone has been lost. However, an X-ray is a 2-dimensional image and does not always give a clear impression of how much bone was lost. A computed tomography (CT) scan is a 3-D image and should give a better impression of how much bone has resorbed around a knee or hip prosthesis. More and more therefore, radiologists try to use CT-scans for this purpose but unfortunately the X-rays used to build a CT scan cannot penetrate the metal from the prosthesis. As a consequence, the CT scan contains “metal artefacts” that mask the bone defects.
Manufacturers of CT scans try to help radiologists by designing software to remove the metal artefacts and improve the image quality. However, different manufacturers use different software, which makes it important to compare between scanners. We therefore tried if we could make a “phantom”, which is an artificial bone with a hip or knee prosthesis inside it surrounded by various-sized bone defects. Such a phantom can be taken to hospitals or manufacturers to assess if the CT scanners correctly visualises the defects.
We managed to make two such phantoms, one for a hip replacement and one for a knee replacement (Fig. 3). Both were very inexpensive (below £50 each) and could be easily transported to other sites. The CT images allowed us to compare image quality between scanners, just as we hoped (Fig. 4). We recently published a paper about the knee phantom, and several people already asked for a copy of the paper so they could also make their own phantom.
Cell therapy for cartilage repair
Our hospital is at the forefront of the clinical use of cell therapy in orthopaedics. We are heading a number of clinical trials in this area, and have collected a large amount of clinical data. Cartilage defect patients are treated using Autologous Chondrocyte Implantation, whereby cartilage cells (chondrocytes) are isolated from a small biopsy and expanded in our OsCell cell manufacturing facility, eventually yielding between 1 and 20 million cells. These cells are implanted into the defect, after which they grow new cartilage. At the moment, we are conducting a clinical trial (ASCOT) to find out if it is best to use cultured chondrocytes, cultured mesenchymal stromal cells or a combination of the two.
The combination of stromal cells and chondrocytes seems attractive because the two cell types are thought to influence each other beneficially. Stromal cells are thought to make the chondrocytes grow (proliferate) faster, amongst others by producing a growth factor known as FGF-1. This would increase the number of chondrocytes in the defect, potentially speeding up healing. On the other hand, chondrocytes are thought to enhance the differentiation of stromal cells into chondrocytes, mainly by producing a growth factor known as BMP-2. This will also increase the number of chondrocytes in the defect. However, having more chondrocytes seems to have no effect at all on the clinical outcome, something we have found in our clinical data and our earlier mathematical models ([1], summarised in the 2011 Annual Report).
To find out if any of these interactions between stromal cells and chondrocytes influence cartilage healing, we adapted our mathematical model of a healing cartilage defect following implantation of chondrocytes [1]. This model took into account such phenomena as cell proliferation, cell migration, production of cartilage and diffusion of nutrients, all expressed as mathematical equations. We added equations representing the mutual influences between stromal cells and chondrocytes. We then used the model to compare how cartilage defects heal following implantation of chondrocytes only (ACI), marrow stromal cells only, or various mixtures of chondrocytes and marrow stromal cells.
Our model predicted that regardless of the type of cells implanted, healing would always occur. However, it predicted that the pattern of matrix density over time clearly differed between implanting chondrocytes only, marrow stromal cells only, or a cell mixture (Fig. 3). After implanting chondrocytes, matrix started to form almost immediately and from then on the mean matrix density in the defect increased linearly with time. After implanting marrow stromal cells it took longer for matrix formation to start, but after around 4 months the mean matrix density had caught up with the chondrocyte implantation case, and from then on the predicted mean matrix density was actually higher (Fig. 3). If implanting a mixture of chondrocytes and stromal cells, the matrix density did not depend on the exact mixture, whether it contained 90%, 75%, 50%, 25% or 10% chondrocytes. However, at all time points average matrix density after implanting a mixture was higher than after implanting either cell type alone (Fig. 3).
An important problem with our mathematical model is that it does not account for differences between patients. Nevertheless, perhaps the results from our ASCOT randomised controlled trial will tell if the model predictions turn out correct.
- Lutianov, M., Naire, S., Roberts, S., & Kuiper, J. H. (2011). A mathematical model of cartilage regeneration after cell therapy. J Theor Biol, 289, 136.
Statistical advice and analysis
Collaborators: The late Prof. James Richardson, Dr Richa Kulshrestha, Mr Naveen Kumar, Mr Shaughn O’Brien, Dr Bushra Naheed, Prof. Sally Roberts, Mr Eric Robinson, Mr Mike Williams.
This year saw our department again put much effort into statistical advice and analysis. This helped in the design and successful funding or ethical applications of several new clinical studies or trials, and in the analysis and publication of several completed studies. One example is a report on a discontinued multicenter clinical trial comparing between bracing and sling to treat shoulder dislocation. We decided to stop the trial early because recruitment had come to a halt, but were still able to draw the meaningful conclusion that bracing would be unlikely to provide the clinical benefits it was supposed to.
Another example is a Cochrane systematic review and meta-analysis of trials on the effectiveness of Gonadotropin-releasing hormone (GnRH) to treat the symptoms of premenstrual syndrome (PMS). We found good evidence that GnRH is indeed effective, but it can only be used for a short time. If longer relief is desired, progesterone must also be taken regularly. Evidence around the effectiveness of GnRH plus progesterone is however weak. Finally, we managed to find predictors of the long-term clinical outcome of Autologous Chondrocyte Implantation (ACI) to treat cartilage defects. We found that the long-term pattern of clinical outcome of ACI could be predicted from three characteristics: the clinical score before the operation, having a patellar or tibial defect, and having two or more defects.
Figure 1: Tendons joined using the Pulvertaft technique (top) and the side-to-side technique (bottom).
Figure 2: Tendons joined using the Pulvertaft technique, clamped in the materials testing machine.
Figure 3: Outside appearance of our hip and knee CT phantom.
Figure 4: CT images of our knee CT phantom from two different scanners. The images from one CT scanner were made without (a) and with (b) using metal artefact reduction software. CT images from another CT scanner (c) show a clearly better image quality.
Figure 5: Predicted mean cartilage matrix density inside a cartilage defect versus time since cell implantation. Four cell implantation cases were compared, namely (1) Chondrocytes only, (2) Marrow stromal cells (MSCs) only, (3) A mixture of 90% chondrocytes, 10% MSCs, and (4) A mixture of 10% chondrocytes, 90% MSCs. A density of 1 represents fully mature cartilage.