Mobility Group
THE WOLFSON CENTRE FOR INHERITED NEUROMUSCULAR DISEASE (CIND).
Inherited muscle-wasting conditions affect approximately 1 in 1000 people, and to date, there are upwards of 50 discrete diseases, each of which is defined by a distinct genetic mutation. Patients commonly present with progressive muscle weakness but the severity and complications vary dramatically between the different diseases. Research at the Wolfson Centre for Inherited Neuromuscular Disease is focused on some of the most severe and devastating muscle-wasting diseases including muscular dystrophies, and the childhood form of motor neuron disease, spinal muscular atrophy. The clinical research team are actively engaged in several pioneering clinical trials and studies involving patients at RJAH and further afield. The internationally-recognised laboratory team, meanwhile, work to find new ways to diagnose and treat inherited neuromuscular diseases. By designing and developing highly specialised research tools and combining these with the use of cutting edge “omics” technology, their research aims to unravel the complexities of disease mechanisms and identify new targets for therapy development. The laboratory team work closely with the clinical team so that scientific advancements can benefit patients as quickly as possible. As a group, they are highly committed to training the next generation of scientists and doctors at RJAH, and work closely with affiliated Universities at Keele, Manchester and Chester, to deliver this.
The Clinical Research Team:
Prof Tracey Willis, Dr Richa Kulshrestha, Mr Nigel Kiely, Dr Yvette Easthope-Mowatt, Mr Nick Emery, Mrs Claire Bassie, Mrs Kerry Jones, Mrs Jenny Moustakas, Mrs Kate Strachan
The Laboratory Research Team:
Prof. Caroline Sewry, Prof. Glenn Morris, Dr. Heidi Fuller, Dr Ian Holt, Dr Le Thanh Lam, Dr Sharon Owen, Darija Šoltić, Emily Storey Affiliate member: Dr Melissa Bowerman (Keele)
The CIND Muscle Team is recognised as a ‘Centre of Clinical Excellence’ for paediatric and adult patients by the Muscular Dystophy UK (MDUK) Charity, reviewed every three years, and was on the 2019 short-list for “Best Team” at RJAH. During COVID 19 they have continued their clinical work and data collection for the national databases for both DMD (Duchenne Muscular Dystrophy) and Spinal Muscular Atrophy patients, with many assessments taking part virtually. A study assessing the usefulness/accuracy of the DMD NSAA (North Star Ambulatory Assessment) was performed via video and assessed independently by two physiotherapists. An abstract has been submitted to the British Paediatric Neurology Association Meeting in Belfast in January 2021. During COVID 19, they have also continued clinical trials and home dosing with virtual and remote visit monitoring and, more recently, face to face visits within guidelines. The team and research nurses are committed to ensuring these studies continue and the patients receive their medication.
The Muscle Team has participated in multicentre studies of the natural history of SMA (SMA Reach project and iSMAc study) and the the Sarepta natural history study of Duchenne Muscular Dystrophy. The pharmaceutical trial, SIDEROS, a placebo-controlled trial with idebenone, targeting mitochondrial function in Duchenne muscular dystrophy, is now in extension phase and one patient has fully completed. The Italfarmaco study is about to enter the extension phase and both are fully recruited.
Last year, friends and colleagues of Professor Caroline Sewry gathered at the Institute of Child Health in London to acknowledge Professor Caroline Sewry’s outstanding contribution to muscle pathology and research in the neuromuscular field over the past 50 years. The meeting was attended by many national and international colleagues from countries that included Wales, Germany, Finland, Sweden, USA and Australia, as well as present and past co-workers from RJAH. Although Prof. Sewry has retired from her position at Great Ormond Street Hospital for Children, she continues to supervise the muscle pathology service at RJAH which she established with Prof. Ros Quinlivan in 1998. In addition, she continues to collaborate with the CIND research team and with the teams at GOSH and ICH.
Legend = ” Participants at the “Sewry-Fest”: Caroline, and husband Steve, are 3rd and 4th from the right.”
Within the Laboratory Research Team, Darija Šoltić passed her PhD with minor corrections, having successfully defend her thesis entitled “Proteomics-based identification and characterisation of spinal muscular atrophy disease pathways” by video call in June 2020. Following publication of work from her thesis in the Human Molecular Genetics journal in 2019, Darija published an invited commentary article in the Neuroscience Insights journal, together with her supervisor, Dr Heidi Fuller. In early 2020, Darija joined the Ruepp group at the UK Dementia Research Institute, King’s College London as a postdoctoral researcher, where she continues her research on neuromuscular disease, but now with a focus on amyotrophic lateral sclerosis.
Spinal muscular atrophy
Prof Tracey Willis and Dr Richa Kulshrestha have been part of the team that secured MRC funding of £968,000 over 3 years for “Segmental Assessment of Trunk Control (SATCo) in SMA” in a collaboration with Manchester Metropolitan University. The Clinical Team has also extended its collaboration with the University Hospital of North Midlands with both adult and paediatric type 2 and type 3 SMA patients (less severe than type 1 SMA) receiving the drug Nusinersen as part of a Managed Access Agreement after approval of this new “wonder drug” by NICE and NHS England (adults to begin in October 2020). Nusinersen is an antisense oligonucleotide drug that targets the SMN2 gene, causing it to make more complete SMN protein to compensate for the missing SMN1 gene in SMA.
Postdoctoral researcher, Dr Sharon Owen, is now in the second year of a laboratory-based project investigating molecular mechanisms involved in different types of spinal muscular atrophy (SMA). This work is funded by Sparks and Great Ormond Street Hospital and is led by Senior Lecturer, Dr Heidi Fuller.
Prior to ‘COVID-19 lock-down’, Dr Fuller and Dr Owen attended the SMA Europe meeting in Evry, Paris (5-7th February 2020) and gave podium presentations on two aspects of their work. Dr Fuller talked about protein changes observed in the hearts of two mouse models of SMA, in particular, the increased presence of a protein called Lamin A/C which may alter the stiffness of the nucleus in these heart cells and hence impact on the ability of the heart to function optimally. Dr Owen’s presentation was based on work investigating protein expression in cells obtained from the skin of SMA patients with varying severity of SMA. It is hoped that this information will provide a means of tracking disease severity, for example, to establish the efficacy of current treatment regimens.
Dr Owen presented an update on the work being carried out on the SMA mouse models at the virtual UK SMA 2020 meeting (10-11th September 2020) suggesting that other key proteins involved in the mechano-sensing machinery of heart cells may be involved in SMA-related cardiac dysfunction. Prof Willis was a member of the Expert Clinical Panel at the same SMA Research meeting.
Facioscapulohumeral Dystrophy (FSHD).
The Clinical Team has successfully completed a pilot study of arm cycling in patients with facioscapulohumeral dystrophy. The results were presented at the UK Neuromuscular Translational Research conference in Newcastle-upon-Tyne and funding has been secured for a PhD studentship to look at this in more detail with collaboration with Keele University. Funding was also secured for another study in this patient group to study biomarkers and their variation with exercise, but is on hold because of COVID 19.
Congenital muscular dystrophy
Congenital muscular dystrophy (CMD) refers to a rare group of muscular dystrophies that become apparent at or near birth. There is no cure, and affected individuals experience progressive muscle weakness and often develop heart disease that can result in sudden death. Although many of the causative mutations are now clear, the ability to identify suitable therapeutic targets is hampered by a lack of understanding of the molecular pathways acting downstream from the defective gene(s) to modulate disease progression. Emily Storey, PhD student within CIND, published a review article in the Neuromuscular Disorders journal, in which she highlighted gaps in knowledge in relation to CMD and areas for future work. Her own research project, which is now in to its second year, involves examining and characterising molecular pathways involved in CMD using a range of laboratory techniques, including proteomics technology. This work will help us to understand why certain genetic mutations lead to congenital muscular dystrophy and will identify potential therapeutic strategies for testing in vivo.
Nesprins and Emery-Dreifuss muscular dystrophy.
Mutations in several different genes (emerin, lamin A, SUN and nesprin-1) can all cause Emery-Dreifuss Muscular Dystrophy, which is characterised by mobility loss involving rigidity at the neck, elbow and ankle, and by cardiac conduction defects. Our study showing the importance of a short nesprin-1 isoform in heart cell nuclei and nuclei associated with neuromuscular junctions has now been published in the open-access journal, Scientific Reports (doi: 10.1038/s41598-019-50728-6). Dr Ian Holt gave a lecture on this work at the 9th UK Nuclear Envelope and Chromatin Organization (NEDCO) Meeting and 2nd International Meeting on Laminopathies held in London in 2019 and Prof Morris was chairman of the session on “New nuclear envelope signalling pathways and mechanisms” at the same meeting . Further studies supported by the Orthopaedic Institute have shown that, while nesprin-1 is located at the nuclear membrane of heart cells, nesprin-2 (the product of a different gene) is located at the region of the heart cell membrane required for cardiac conduction (the “intercalated disc”) where it may be dependent on the Emery-Dreifuss muscular dystrophy protein, emerin. This is the first evidence for a separate location of the two nesprins.
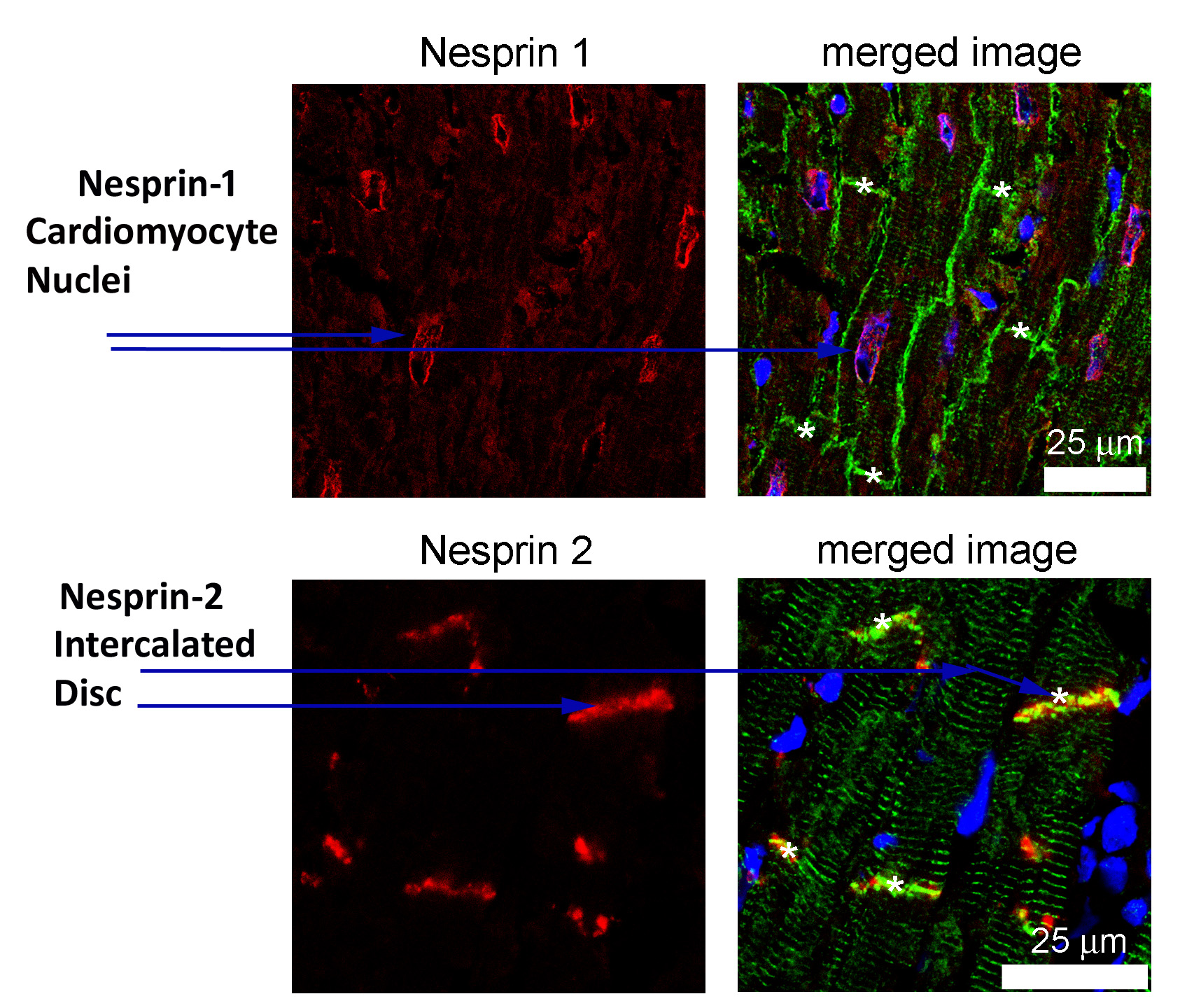
Legend = ” Emery-Dreifuss muscular dystrophy: the two forms of nesprin are found at different locations in the heart.”
Popeye Proteins and Cardiac Conduction.
Dr Ian Holt has continued to characterize monoclonal antibodies (mAbs) against Popeye (POPDC) proteins by mapping exactly where the mAbs bind to the protein antigen sequence in collaboration with Prof. George Baillie’s group at Glasgow University.
Mutations in Popeye proteins can cause a form of limb-girdle muscular dystrophy with conduction defects, similar to Emery-Dreifuss muscular dystrophy. In a collaborative project with Thomas Brand of the National Heart and Lung Institute (London), funded by the British Heart Foundation, Dr Ian Holt has shown an interaction of both POPDC1 and POPDC2 with both cardiac actin, annexin A5 and XIRP1 (Xin actin-binding repeat protein 1) at important locations for cardiac conduction (intercalated discs and transverse tubules). Both XIRP1 and POPDC1/2 are proteins known to be associated with heart pathology and cardiac conduction defects. This work has been submitted for publication.
The MDA Monoclonal Antibody Resource.
Monoclonal antibodies produced by CIND and the cell lines that produce them have been given a safe and permanent home in the Developmental Studies Hybridoma Bank (University of Iowa). These mAbs have played a major role worldwide in clinical trials of new treatments for the muscular dystrophies. Dr Le Thanh Lam is continuing to respond to antibody requests from academic researchers worldwide and, in August 2020, we began to support the cost of this service by making a small charge to recipients. Prof. Morris initiated a new collaboration with the University of California at Irvine in 2020 aimed at developing novel antibody-based treatments to depress inflammation in Duchenne Muscular Dystrophy.
Nemaline Myopathy
Nemaline myopathies are named after a structural body seen in muscle biopsies. Defects in several genes are known to cause various clinical forms of nemaline myopathy. One of the most common genes affected codes for a protein called nebulin, one of the largest proteins known in the human body. The team at RJAH have developed antibodies that specifically recognise two different forms of nebulin. These are expressed at different times during muscle development and may have a role in particular types of muscle fibres . Research by the group is examining whether different forms of nebulin might relate to the differential involvement of certain muscles in particular diseases of muscle, including nemaline myopathies. Mutations in some of the protein partners of nebulin also cause nemaline myopathy and we are testing the possibility that the partnership is with only one of the two nebulin forms.
ORLAU – ORTHOTIC RESEARCH AND LOCOMOTOR ASSESSMENT UNIT
Clinical Activity
ORLAU is a busy department and there has been plenty going on this year. We continue to see a steady stream of patients for analysis of their movement or supply of assistive devices and orthotics. Over the years we have built up a reputation for helping patients to walk better, which inevitably means studying legs. More recently our upper limb service has been growing and we hope to expand the number of clinics in the future so we can help more adults with their arm function. We are continuing to develop our gait analysis services too and saw our first amputee patient recently, working with the Artificial Limb and Appliance Service (ALAC) at Wrexham.
Our rehabilitation engineering team have also been busy, developing and testing new designs to improve our patient devices. The team also look after ORLAU’s services in the community. Matthew Hardy has been busy running our Community Standing Frame Service, having taken over from Graham Richards in the Summer of 2018. We have also introduced new digital technology to our transportable gait laboratory.
All this clinical work feeds into our research plans for the future.

Locomotor assessment sensors

Research is vital to the team
Education
We are very keen to pass on our expertise and learning to others and this work has continued over the last year. As a department we support national training initiatives for physiotherapists and clinical scientists. We have also put a bespoke course together for Matthew Hardy to support his new role in the community.
Individual members of staff are involved in teaching nationally and internationally but in 2018 we came together to run our first gait analysis course in some years. The course was organised by Sarah Jarvis under the umbrella of the Orthopaedic Institute and we received excellent feedback from our delegates. The same course ran again in June 2019 and we are planning a new course, in contracture management, for the Autumn, led by engineer Keith Miller and physiotherapist Will Bromwich.
ORLAU technical staff have been developing their expertise in writing computer code over the last year. Early in 2019 we were awarded a grant by CMAS (the gait analysis society covering the UK and Ireland). The grant has allowed us to set up a national programme for sharing and learning coding, aimed at staff working in gait labs. That programme is being run by Dimitra Blana, a collaborator of ours from Keele University.
Movement Analysis
In January 2019 we welcomed Dr Raphael Gross to ORLAU. Raphael is a rehabilitation doctor from Nantes in France and he joined us for a 6 month fellowship to study muscle co-ordination in the upper limb. His costs were covered by the Orthopaedic Institute, funding which made the visit possible. He and the ORLAU team have been busy collecting and analysing data from lots of volunteer children. We are looking forward to seeing the results and applying his approach to our patients with hemiplegia.
The Institute have also provided key funding for our postgraduate students. PhD student Shallum Sardar is looking at the calf muscle, in particular how the morphology of the muscle changes after treatment. He has been working with Myurah Nathan, our clinical scientist trainee. Myruah looked at the same children for her MSc project but she was interested in studying the muscle using imaging. She worked with consultant radiologist Naomi Winn to collect ultrasound elastography data. Shear Wave Elastography is an imaging technique which aims to quantify tissue stiffness. Myurah successfully completed her research in the Spring, whilst Shallum continues to collect data.
Another PhD student, Mohammad Alshehab, has been working hard to gain ethical approval for his study, assessing upper limb function in cerebral palsy. Mohammad hopes to quantify the benefits of using Lycra garments. It is great to see our growing upper limb clinics also reflected in our research. Along with Raphael and Mohammad’s work we have been successful in gaining funding from the EPSRC for a study entitled ‘Personalised approach to restoration of arm function in people with high-level tetraplegia’, led by Dr Ed Chadwick from Keele University, working with the Trust’s Simon Pickard.
Title

ORLAU Ankle Contracture Correction Device (CCD)
Rehabilitation Engineering
The Rehabilitation Engineering team have seen a number of significant developments this year. Industrial partners are particularly important for our development work and this year we have developed a new relationship with Rehabilitation Manufacturing Services (RMS) Ltd, who now supply us with the Grillo range of devices, manufactured by Ormesa in Italy. We have worked hard behind the scenes to develop a relationship that has resulted in them providing a small ‘stock’ of these devices, which we can use to assess and supply the patient, with RMS charging for them, as we issue them. This has a dramatic effect on the delivery time for the patient, as they can often take the frame home immediately. Many of our other devices have much longer lead times for delivery, typically 6-12 weeks, so not only can that cause an inevitable delay, it also results in a further patient appointment. The Grillo widens our offering for walking device assessments.
Last year we reported the early development of a donning/doffing lever for our ankle contracture correction device (CCD) in collaboration with Ricoh Rapid Fab Ltd. The original CCD was designed and developed in ORLAU. We are now supplying the new levers routinely to all ankle CCDs supplied through ORLAU. In the coming year our intention is to explore opportunities to further improve and commercialise this device with the help of an appropriate industrial partner.
By using money provided by a charitable donation, we are currently developing a safe and simple method of detaching the upper and lower sections of an Adult ORLAU Standing Frame. This is a much needed development as we see numerous patients with these types of frames who struggle to transport them due to their cumbersome size and weight. By safely disconnecting the two halves of the device transportation to and from clinic, as well as storage at home should be made much easier for the patients and their families.
Engineer, Simon Marchant is also developing a bespoke bracket to help us attach head supports to our range of standing frames. The current bracket is a commercial one that we adapt to suit our needs but the time has come to develop our own, that meets the specific needs of our patients.
In conclusion ORLAU is continuing to develop clinical services, alongside teaching and research. We are always interested in growing our knowledge and have new projects lined up for 2020, including work in the social sciences and a detailed study of the subtalar joint in cerebral palsy. We will look forward to giving more details about these new projects next year.

