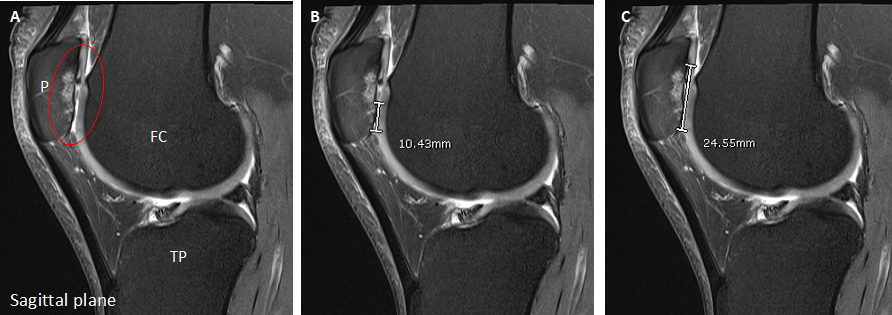
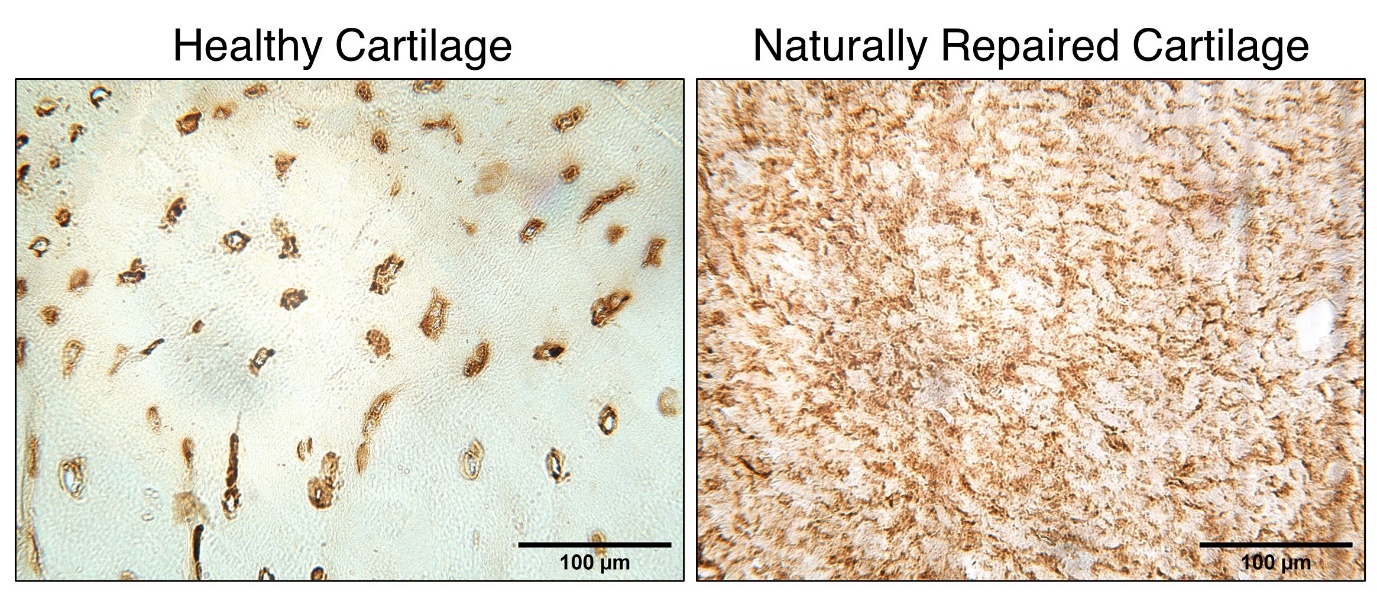
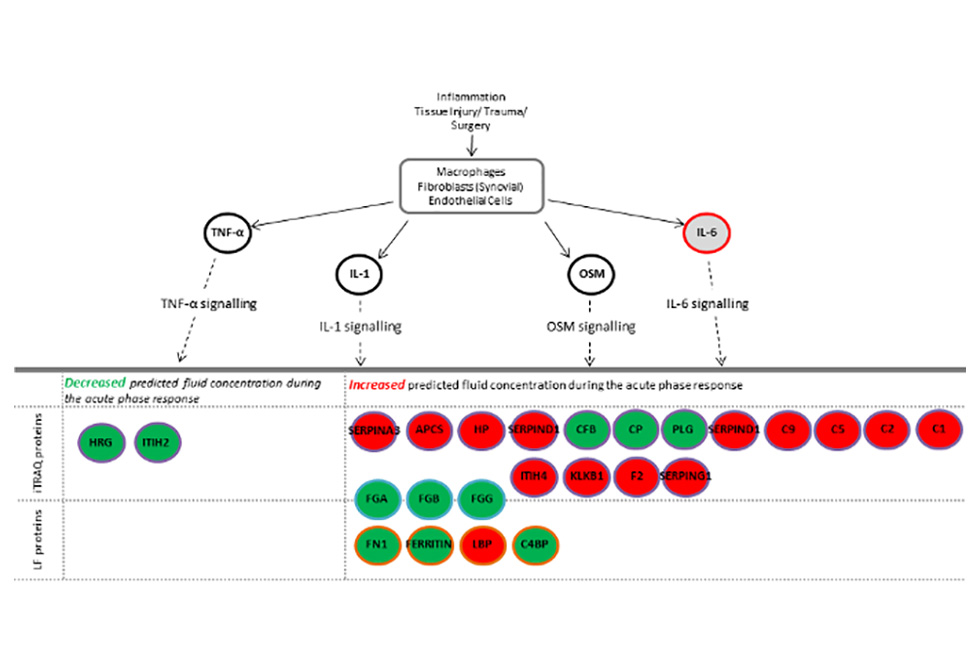
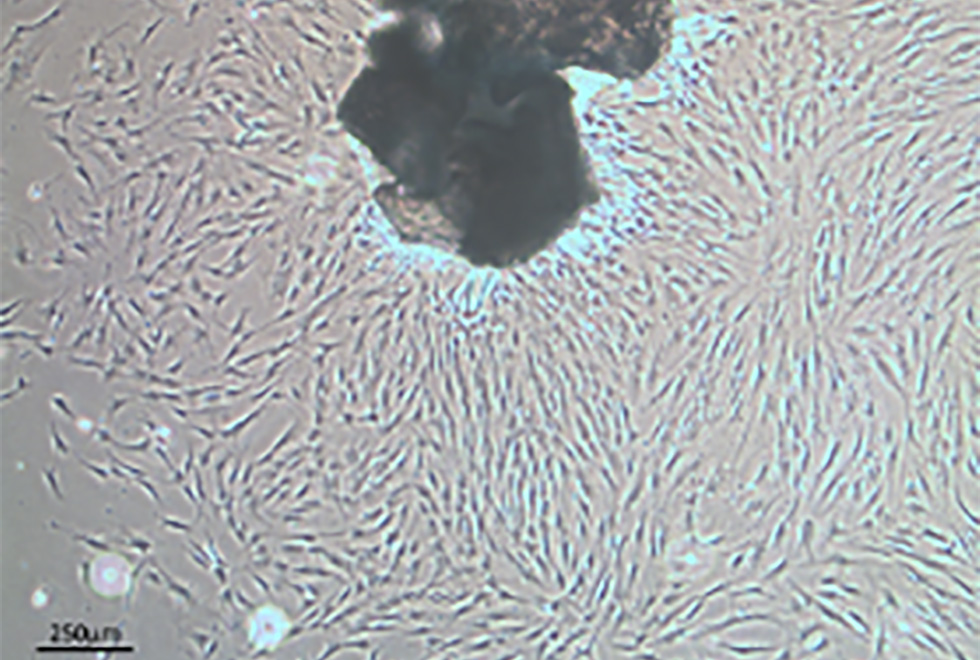
Cell Therapies
CARTILAGE RESEARCH GROUP
Members of the Research Team: Sally Roberts, Karina Wright, Claire Mennan, Jan Herman Kuiper, Helen McCarthy, Johanna Wales, Charlotte Hulme, Sharon Owen, John Garcia, Mike Williams, Barbara Linklater-Jones, Paul Harrison, Nikki Kuiper and PhD students, Jade Perry, Tim Hopkins, Jingsong Wang (Kobe), Jessica Sykes and Angus Armstrong-Twigg (Cardiff).
Clinical Support: Messrs Peter Gallacher, Paul Jermin, Drs Bernhard Tins, Andrea Bailey, Professor Iain McCall and all surgeons in RJAH
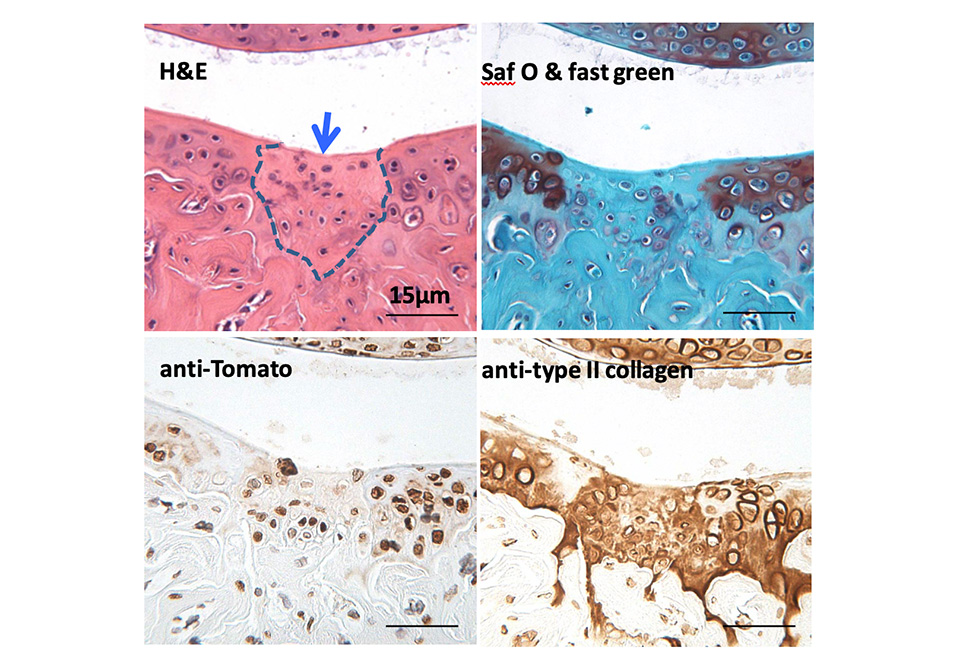
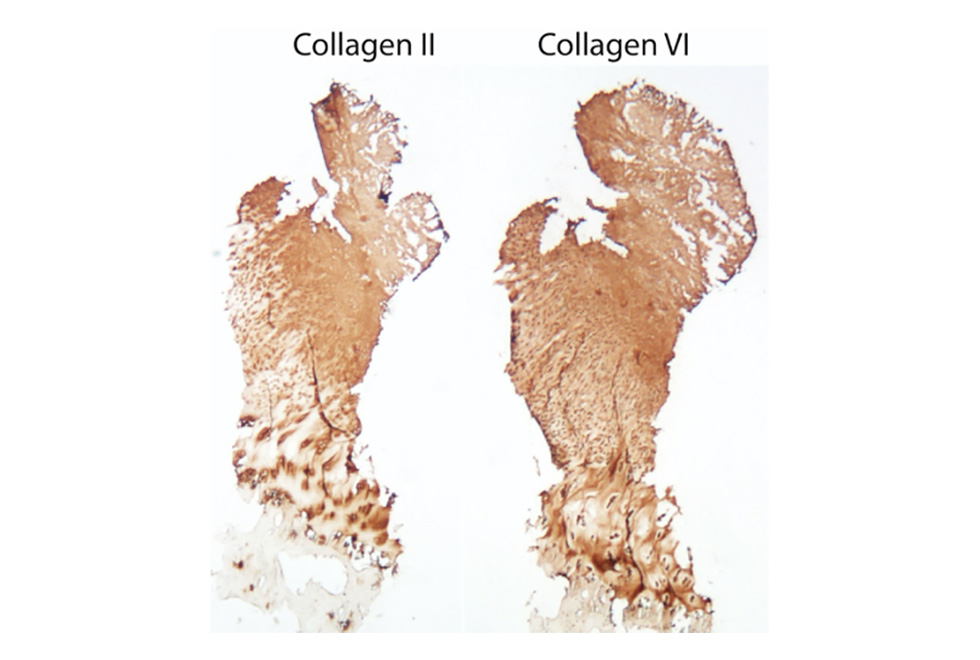
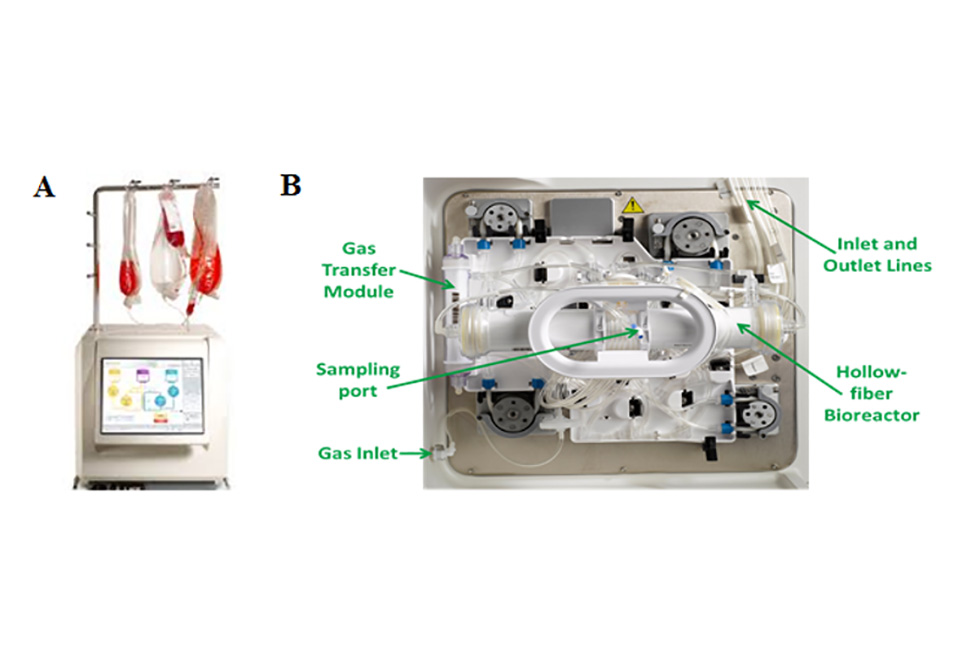
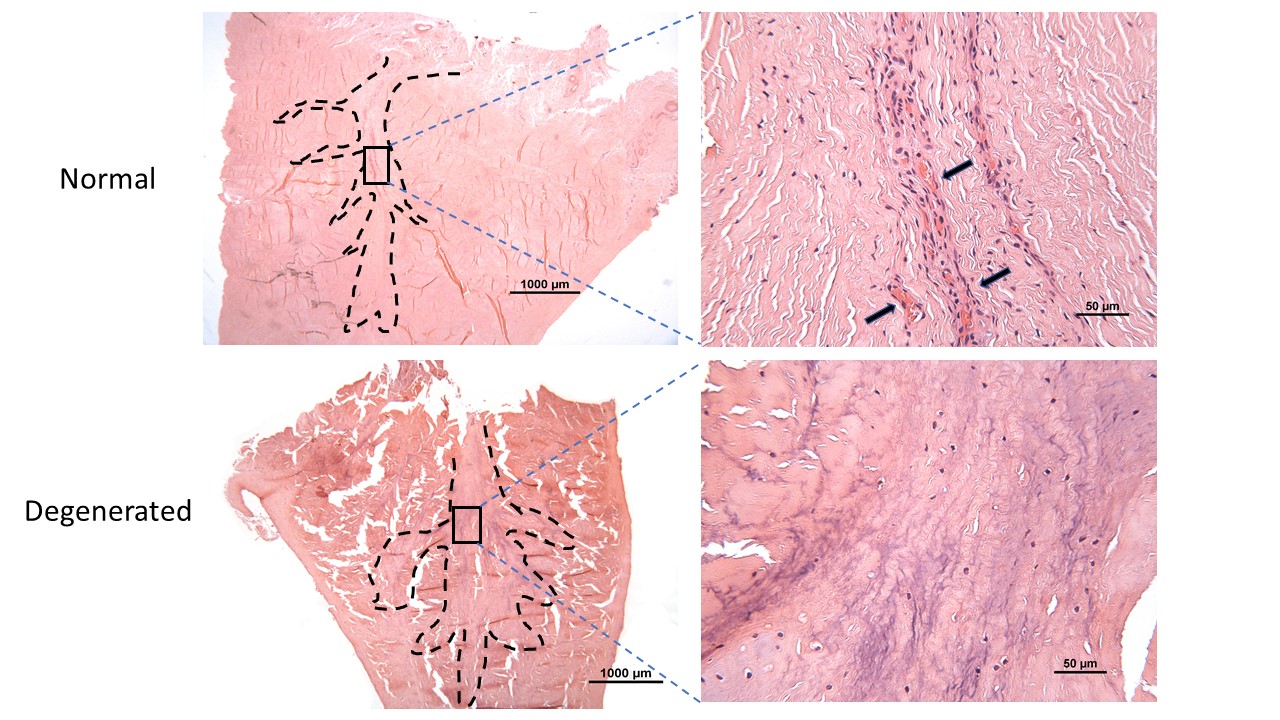
Scientists based in the Leopold Muller Arthritis Research Centre and TORCH laboratories continue the long history of the RJAH Orthopaedic Hospital seeking to understand joint disorders and ways of treating them. The group has continued over the last 12 months focussing on particular areas such as chemical assays of ‘biomarkers’, growing cells in the laboratory and seeing how we can influence their behaviour and different aspects related to cell therapy. We are very fortunate in RJAH to be able to work closely with clinicians and surgeons who themselves are often very interested in research. Much of the work involves analysing material from patients undergoing surgery which is being removed (eg an arthritic knee joint) and would otherwise be thrown in the bin. If patients are happy to allow us and the work is approved by a Research Ethics Committee, then we can put this ‘surgical waste’ to good use. For example, biomarker studies can help unravel different chemical pathways involved in diseases and also to in see if sub-groups of patients can be identified who may suit a certain treatment more than others. The cell therapy work includes researching if we can produce ‘off-the-shelf’ cell products which could be much easier for the patient and surgeon to use, as well as being cheaper that growing patients’ own cells in the laboratory as happens now.
Most research is funded by grant applications, for example, from the Medical Research council or charities such as Versus Arthritis. We are however very grateful to the Orthopaedic Institute as the large grant-giving bodies usually require preliminary data, and the projects that the Institute fund are wonderful for obtaining this and help to get the larger grants.
Cell Therapy Projects